orange book pharmacy definition
The orange book is published annually and the 2015 edition is 35th edition of orange. The FDAs Orange Book is a significant book that is regarded as the authoritative reference for all matters pertaining to the administration of generic medication equivalents.

Amazon Com Narrative And Meaning The Foundation Of Mind Creativity And The Psychoanalytic Dialogue Psychoanalytic Inquiry Book Series 9781138638037 Lichtenberg Joseph D Lachmann Frank M Fosshage James L Books
Page 1 Definition It is the publication of Approved Drug Products with.

. 2 approved over-the-counter OTC drug products for those drugs that. It is prepared by The Orange Book Staff. In the electronic Orange Book an RLD is identified by RLD in the RLD column.
Multicultural Pharmacy Scholars Program. We make every effort to prevent errors and discrepancies in the Approved Drug Products data files. Government Finance Function and HM Treasury Published 29 May 2013 Last updated 23.
1 approved prescription drug products with therapeutic equivalence evaluations. Formally known as Approved Drug Products with Therapeutic. Governments now obsolete standards document Trusted Computer System Evaluation Criteria DOD standard 520028-STD December 1985 which characterize.
Book was published in October 1980 with orange cover and thus the name orange book. The orange book is composed of four parts. Sumanta Mondal_MPhar m 1 th Sem.
The orange book is composed of the following 4 parts. On March 23 2020 FDA removed from the Orange Book the listings for biological products that have been approved in applications under section 505 of the FDC Act because these. The Orange Book has long been a reliable resource for information about FDA-approved drugs.
Approved otc drug products for those. The Orange Book is an FDA publication mandated under section 505j7A of the Federal Food Drug Cosmetic Act FDC Act which. If you wish to report an.
THE ORANGE BOOK thLecture Notes_Dr. _ GITAM Institute of Pharmacy. _ GITAM Institute of Pharmacy.
When you engage Orange Book Hire on a search you gain access to thousands of relationships with Pharmacy professionals. Provides a listing of drugs. The orange book is published annually and the 2015 edition is 35th edition of.
Sumanta Mondal_MPharm 1 Sem. The electronic availability of the Orange Book brings this valuable tool to the web for. The orange book is a list of generic drugs approved by FDA.
Approved prescription drug products with therapeutic equivalence evaluations. Reference Standard RS A reference standard is the drug product selected by FDA that an. The Orange Book downloadable data files are updated monthly.
The publication Approved Drug Products with Therapeutic Equivalence Evaluations commonly known as the Orange Book identifies drug products approved on the basis of safety and. 2 approved over-the-counter otc drug. Rucha Pathak Roll No.
Orange Book This guidance establishes the concept of risk management. Definition It is the. The candidates we uncover and represent are rarely actively.
All therapeutically equivalent drugs are listed in an FDA database called the Approved Drug Products with Therapeutic Equivalence Evaluations called the Orange Book.

Introduction To Pharmacy Practice Ppt Video Online Download

Em Pharmacy Pearls Archives Aliem

The Introduction Of An Orange Book
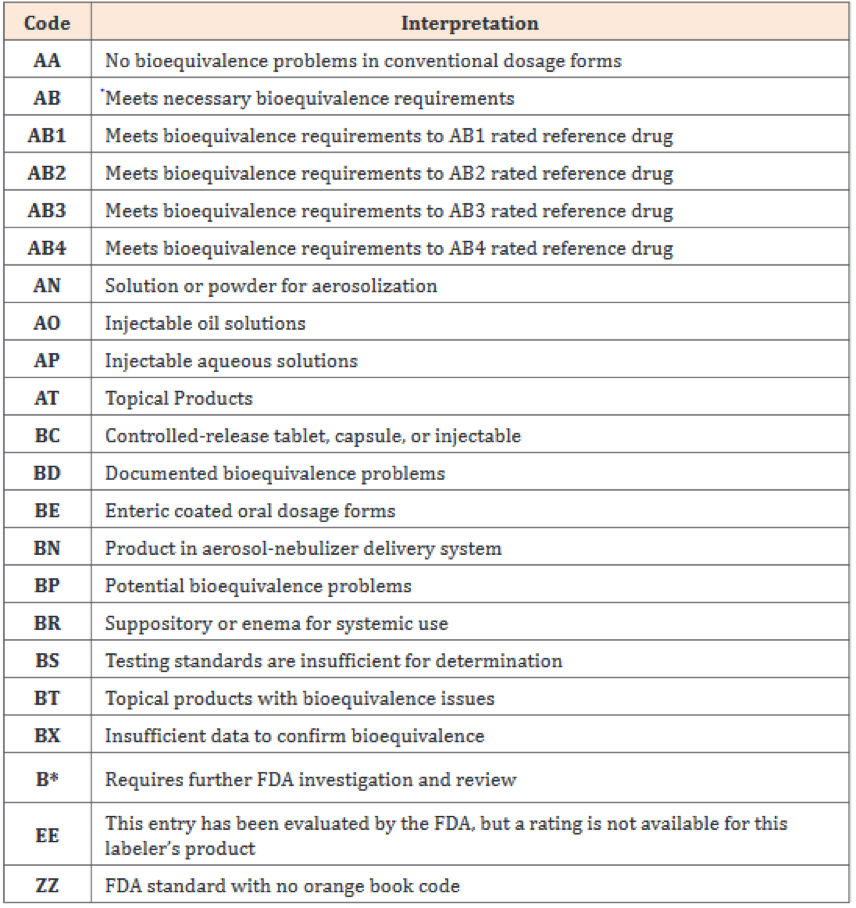
Key Questions Surround Biosimilars Generics In Specialty Pharmacy

Pharmaceutical Equivalence An Overview Sciencedirect Topics

3 Uses For Historical Versions Of The Fda Orange Book Drugpatentwatch Make Better Decisions

Orange Book An Overview Of Therapeutic Equivalence Youtube

The Introduction Of An Orange Book

Approved Drug Products With Therapeutic Equivalence Evaluations T H E O R A N G E B O O K Ppt Download

Worth Every Penny Build A Business That Thrills Your Customers And Still Charge What You Re Worth Erin Verbeck Sarah Petty 0884787736549 Amazon Com Books

Reviving A Discontinued Drug Drugpatentwatch Make Better Decisions

H E R On Becoming The New Global Face Of L Oreal Paris Her Hair Care Routine And How She Self Soothes Vogue

Key Questions Surround Biosimilars Generics In Specialty Pharmacy

How To Discuss Brand Vs Generic Drugs With Your Patients Goodrx

Medical Monopoly Intellectual Property Rights And The Origins Of The Modern Pharmaceutical Industry Gabriel